About Regulatory Professionals
The regulatory professional serves a vital role in ensuring safe and effective healthcare products for patients and healthcare providers. The regulatory profession is vital in making safe and effective healthcare products available worldwide. Individuals who ensure regulatory compliance and prepare submissions, as well as those serving in clinical affairs or quality assurance roles, are all considered regulatory professionals.
Regulatory professionals are employed in industry, government and academia and are involved with a wide range of products, including:
Pharmaceuticals
Medical devices
In vitro diagnostics
Biologics and biotechnology
Artificial intelligence/digital health
Combination products
Quality
Clinical
The regulatory professional's roles and responsibilities often begin in the research and development phases, moving into clinical trials and extending through premarket approvals, manufacturing, labeling and advertising, and post-market surveillance.
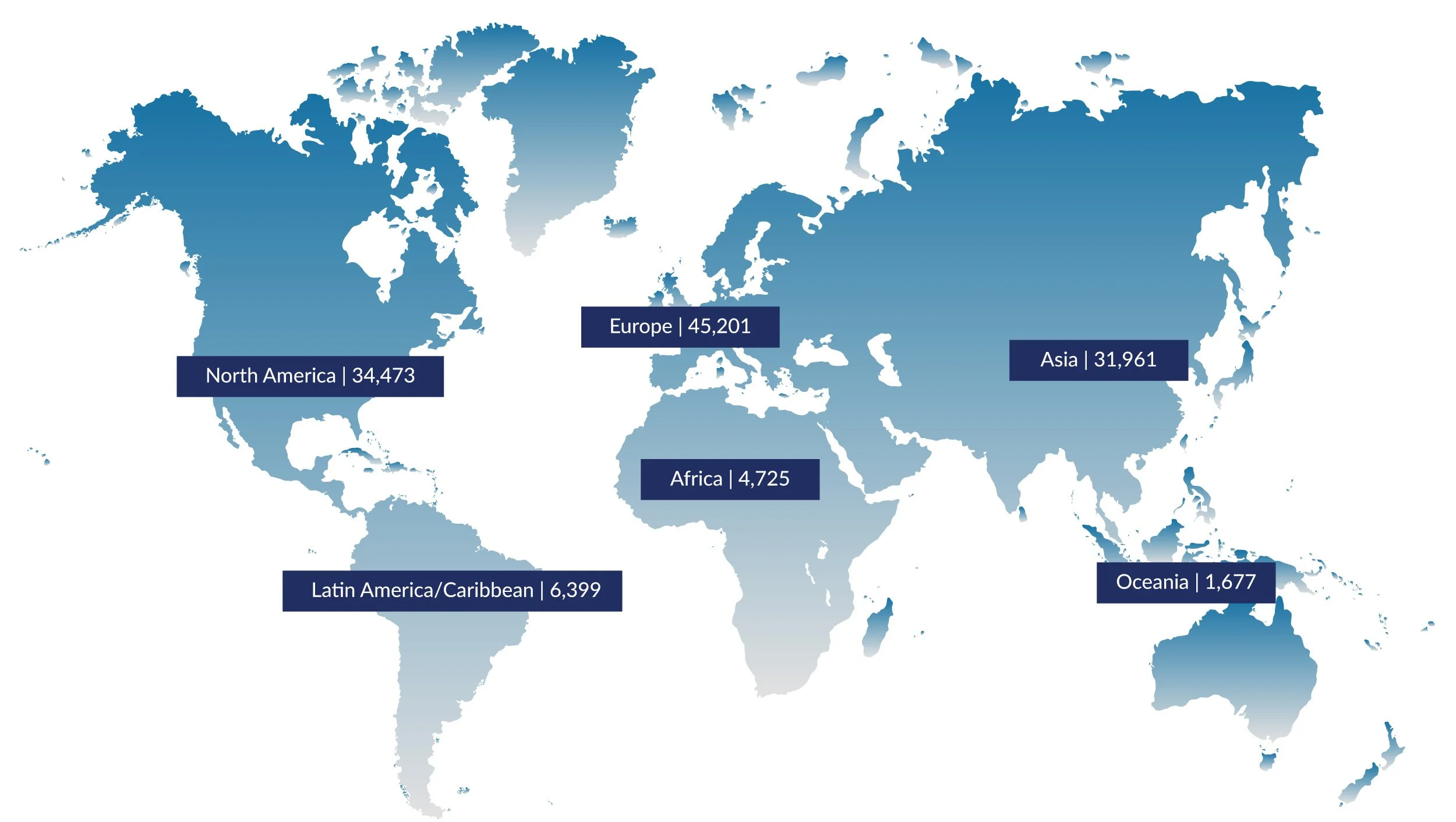
Regulatory Affairs Professionals Globally
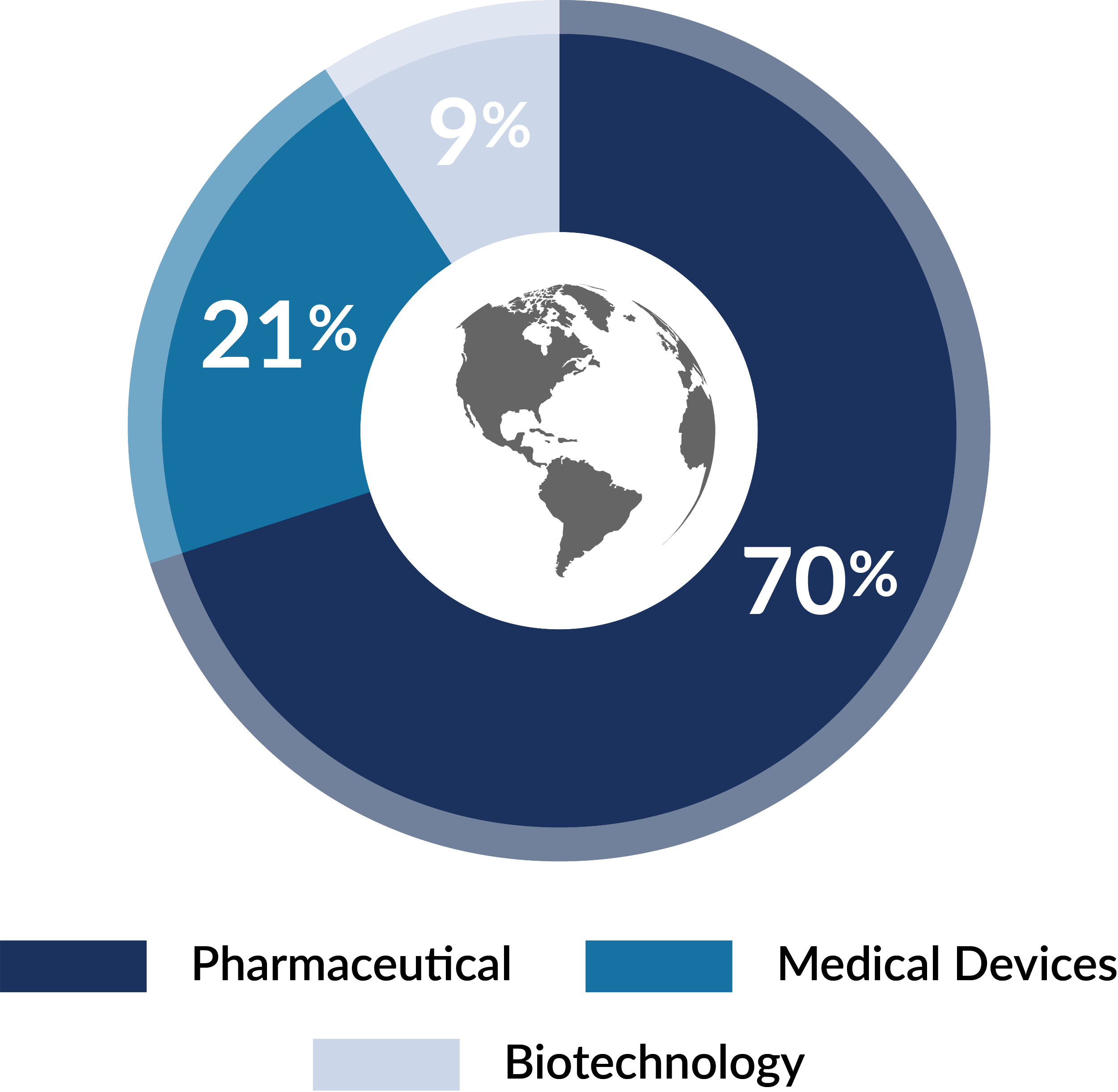
Global Regulatory Affairs Professionals by Sector
Source: RAPS Global Regulatory Affairs Professionals Workforce Report (May 2024)
About RAPS
The Regulatory Affairs Professionals Society (RAPS) is the largest global organization of professionals involved with regulatory and quality for healthcare products, including medical devices, pharmaceuticals and biologics, diagnostics, and digital health. Founded in 1976 as a neutral, nonprofit organization, RAPS supports and elevates the regulatory profession with education and training, professional standards, publications, research, networking, career development, and other valuable resources. RAPS is home to the Regulatory Affairs Certification (RAC), the only post-academic professional credential to recognize regulatory excellence.
RAPS is headquartered in suburban Washington, D.C., with chapters and affiliates worldwide. To learn more—or to join RAPS—please visit www.RAPS.org.
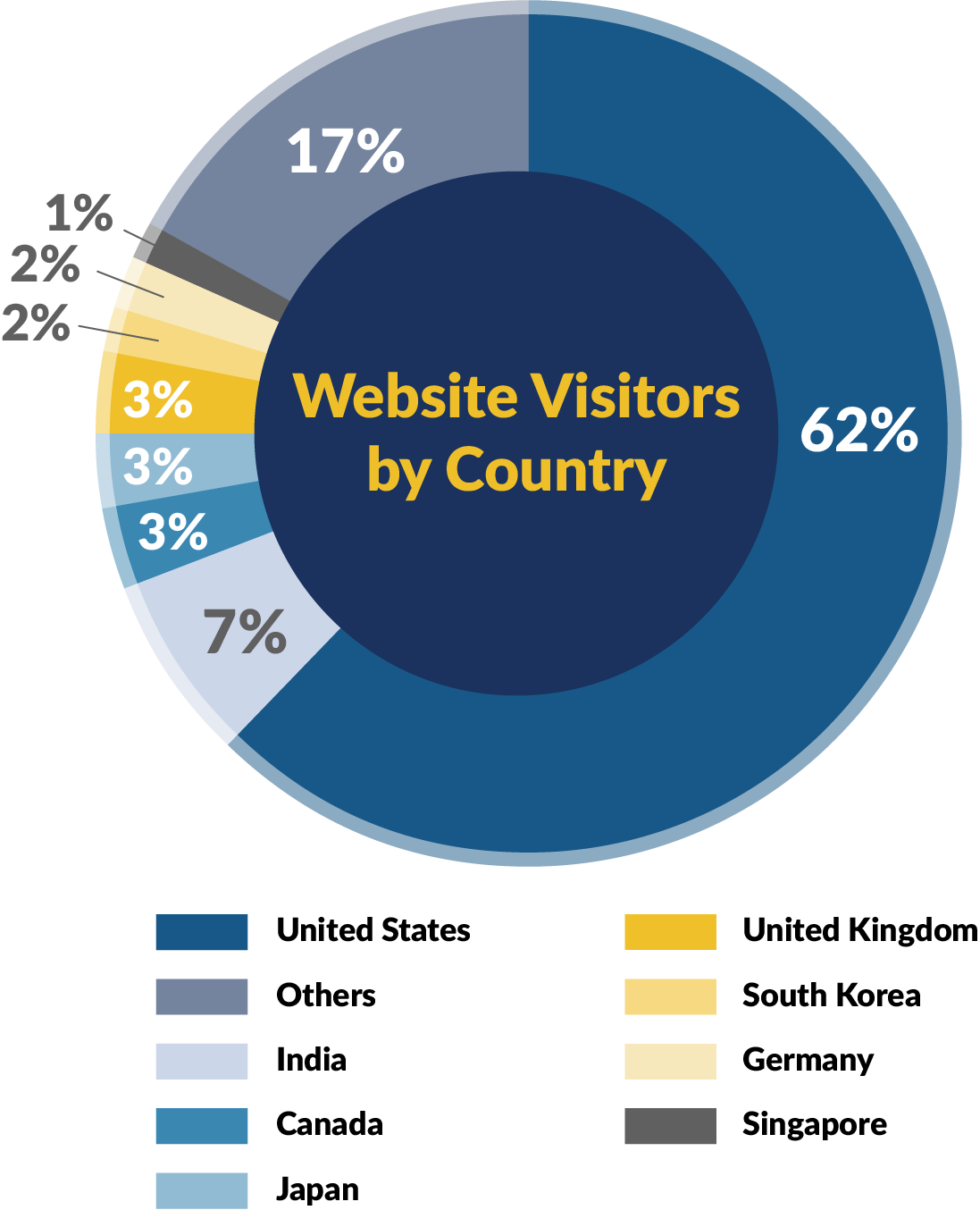
Membership Demographics & Insights
Purchasing Intentions
Source: RAPS Membership Survey (August 2023)